3 min read
Blog: Stability’s Role in the Drug Formulation Process
From molecule discovery through product release to market, quality attributes are carefully analyzed to support product success and protect patients.
After expounding on the importance of efficacy and degradation in the previous blog, this fourth edition of the stability series will answer the most common questions people have about storing medicines and explain how storage plays a direct role in a product’s efficacy, along with which studies are used to measure efficacy.
Is it necessary to read the storage conditions listed on the label? Could where I keep my medicine damage it?
Yes! How medicine is stored influences its efficacy and safety. Remember to always follow the recommended storage conditions for the best use of a finished pharmaceutical product.
Heat, humidity, and light are all responsible for the degradation of products. Regulation committees, such as the Federal Drug and Food Administration (FDA), ensure that proper precautions are taken in order to maintain consumer safety. Establishing product integrity within different climate zones is essential because just the slightest change in temperature and humidity could affect medicines.
Stability storage and testing studies are performed to simulate climatic effects. The studies are based on where the products are going to be sold. Knowing all the ways a finished product or active pharmaceutical ingredients (APIs) could be affected by degradation is crucial in the storage of these products. From those studies, Alcami is able to establish shelf life of the medicine, determine the best way to store the medicine, and ultimately help ensure the safety of the consumer.
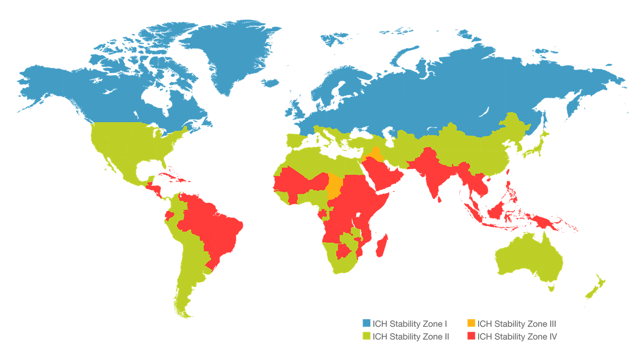
The International Conference on Harmonisation (ICH) distinguishes four worldwide climate zones as followed:
Zone I: temperate
Zone II: subtropical, with possible high humidity
Zone III: hot/dry
Zone IV: hot/humid
Accelerated studies, or forced degradation, are used to predict the shelf life of a product. Scientists speed up the processes and rate of decomposition/degradation by increasing the temperature and/or humidity of environmental conditions for a brief period of time. These accelerations demonstrate the impact on the medicine if introduced to extreme conditions for a short period of time. Overall, this gives a general overview of the different properties of degradation including physical, chemical, and microbiological.
Freeze-thaw studies determine whether the formula will remain stable under various conditions. Freezing is a common processing step used to maintain the stability and quality of a drug substance during the development and production of the medicine. The finished product/APIs are put through a series of rapid changes that could be encountered during shipping and handling of samples. The sample goes through a cycle of freezing conditions, usually around negative 20°C, and placed in higher temperature conditions, typically 25°C – 45°C, then tested. This cycle will occur over a span of a couple of days/weeks. The testing looks for any significant differences of the overall product throughout the entirety of the study.
Below is the ICH guidelines table for the different study parameters and the minimum time requirements needed to support the data. Please note that relative humidity is referred to as RH.
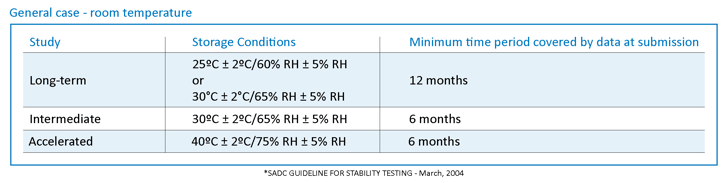

These parameters are set in place so pharmaceutical companies can establish strong data to support optimal storage conditions based on climate zones. Simulating these conditions and seeing how, over time, the product efficacy is affected is the purpose of stability.
In closing, remember to always properly store medicine in accordance with the manufacturer’s directions. Alcami is an industry leader in stability expertise and capabilities offering full ICH storage conditions, redundant facility capacity and any unique studies required like in-use, photostability, and freeze/thaw.
3 min read
From molecule discovery through product release to market, quality attributes are carefully analyzed to support product success and protect patients.
2 min read
Can you answer any of these questions?
2 min read
In the WHO Technical Report Series, No. 953, 2009 Annex 2 Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products...