5 min read
Blog: Successful Methods for Obtaining Orphan Drug Approval
Signed into law on January 4, 1983, the Orphan Drug Act (ODA) allows for granting special status to a drug or biological product (drug) to treat a...
3 min read
 Alcami
:
Oct 30, 2018 1:30:00 PM
Alcami
:
Oct 30, 2018 1:30:00 PM
Controlled drug substances are scheduled based on medical use in treatment, potential for abuse, and dependence caused if abused— the lower the control schedule the more potential for abuse. The process to obtain approval from the Drug Enforcement Administration (DEA) for Schedule I-V controlled substances and List I chemicals licensing involves intricacy and a detailed timeline of approvals. This article will discuss the background of controlled substance applications and how to successfully work with the DEA.
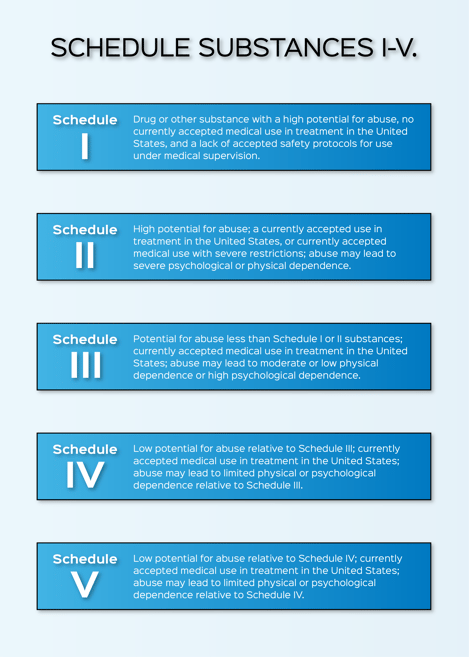 Reference: https://www.dea.gov/
Reference: https://www.dea.gov/
Every year the United States sets Aggregate Production Quotas (APQ) for Schedule I, Schedule II, and Pseudoephedrine, Ephedrine, and Phenylpropanolamine (PPA) substances. The quota is based on the Controlled Substance Act (CSA) number. List I chemicals are also included because of the Combat Methamphetamine Epidemic Act (CMEA). All bulk active pharmaceutical ingredient (API) manufacturers that produce controlled drug substance API, manufacturers and packagers of Schedule I and II controlled substances, and manufacturers and importers of Pseudoephedrine, Ephedrine, and PPA must apply for quota. The request and approval/denial of quota can take six to eight weeks to process and bulk manufacture API can take six to nine months to process. This is important to consider when forecasting project timelines.
Quota requests have deadlines due for the next year’s forecast for manufacturing and packaging by April 1 of each year with DEA response by mid-December. Bulk API manufacturing quota requests should be made before May 1 of each year with the DEA response expected by mid-December as well. Keep in mind that the earlier the quota is requested the better. (Quota Applications, 2018)
Manufacturers assigned to individual manufacturing quota may abandon their right to manufacture quota at any time by filing with written notice via DEA website.
The notice should include:
The administrator may allocate the amount amended to the other manufacturers in proportion to their respective quotas. For the DEA, a registration is required to import and export controlled substances and controlled reference standards to and from abroad.
The approval process consists of eight steps that must be adhered to in order to file successfully. When partnering with a contract development and manufacturing organization (CDMO), it is important to select one that has vast knowledge and experience with filing controlled substance licenses with the DEA. Involve the DEA expert from the start to protect your product from delays and to fully understand the timelines needed for approval.
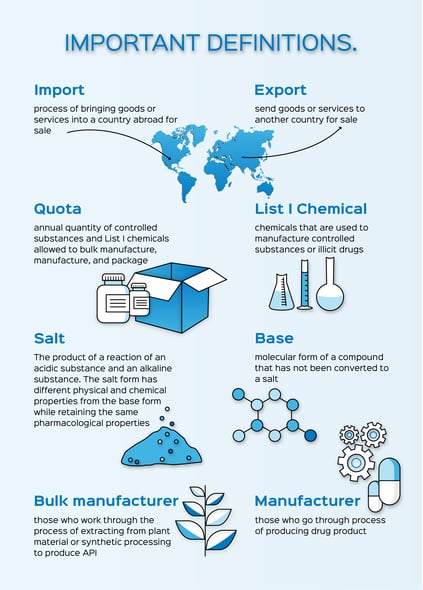
Reference: https://www.dea.gov/
The export process is similar except the CDMO is required to receive an import permit from the importer to send the product, even for small quantities.
Both of these processes can take at least two months. The process takes this long in order to obtain the required information for various forms to apply for import/export, additional permits/certificates, and the product needs customs approval. For customs, registrations and package descriptions must align, and multiple checkpoints occur.
Thank you for your interest in learning more about Schedule I-V controlled substances and List I chemicals processes for quotas, imports, and exports. Alcami has the capacity and expertise to support your controlled substance drug product timelines and milestones for API, sterile, and oral solid dosage (OSD) forms. Our DEA subject matter experts are happy to help you navigate the process with the correct manufacturing requirements and compliance as well as DEA audits.
About the Author
Jjasmin Zorrilla, MPM, has over 20 years of experience in the lifecycle of product development to ensure all products meet regulatory and compliance requirements for controlled substances in the pharmaceutical industry in the United States and Canada. Her controlled substances expertise spans across oral solids, parenteral, liquids, trans-dermal, sublingual, preformulation, formulation, analytical research & development, product development, generic, and branded. Jjasmin holds a Bachelor of Science degree in Business Administration from Berkeley College, a Master’s degree in Project Management from Keller Graduate School of Management of DeVry University, and is currently pursuing accreditation for supply chain management.
https://www.luc.edu/media/lucedu/ors/pdfsanddocs/research/FAQ_CS.pdf
https://www.deadiversion.usdoj.gov/schedules/index.html
Controlled Substance Schedules. (2018, October 1). Retrieved from DEA: https://www.deadiversion.usdoj.gov/schedules/index.html
5 min read
Signed into law on January 4, 1983, the Orphan Drug Act (ODA) allows for granting special status to a drug or biological product (drug) to treat a...
7 min read
Supporting a compound from discovery to clinical phases and potentially commercial phases requires an immense amount of time, energy, and money....
4 min read
According to the US Orphan Drug Act of 1983, a rare disease is defined as a condition that affects fewer than 200,000 people. The term orphan disease...