2 min read
In-Use Stability Testing: Providing the Right Instructions
In the WHO Technical Report Series, No. 953, 2009 Annex 2 Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products...
The protocol design, execution, and reporting of the confirmatory photostability for API and pharmaceutical products will be discussed. ICH guidance Q1B presents a straightforward flowchart that initiates with the direct exposure of the dosage form. To initiate a study, a protocol should be written based on the steps outlined in the flowchart. However, not every step in the flowchart is needed.
At this point in product development, it is very likely that the photo sensitivity of the API is known. If the API is photo-labile, a prudent step would be to write the protocol to direct exposure of the product, and exposure of the product in its immediate packaging would occur simultaneously. Not only would this save time, but would demonstrate the effect of the immediate pack has in protecting the product from photo-degradation. On the other hand, if the API is photo-inert, the direct exposure of the product may be the only exposure required. As stated in the guidance, each exposed foil-wrapped dark control sample should be exposed and tested.The protocol must include the regiment of testing and the specifications. These two items should be identical or very similar to that of the long-term product stability studies. The testing should include the physical, chemical, and performance testing that is performed for the long-term stability studies, however, microbiological testing is not needed. At Alcami, the photostability study is commonly scheduled such that the testing of the photostability samples is performed with one of the scheduled pulls from the long-term studies. This eliminates the need for additional assay set-ups.
The protocol should also include the type of exposure, exposure limits, and the temperature range of the photostability chambers. For example, the following statements should be included: “Product and dark control should be exposed to both cool white fluorescent light and near UV radiation. The cool white fluorescent light should have a spectral distribution similar to ISO 10977 and the near UV radiation should have a spectral distribution from 320 nm to 400 nm with a maximum energy emission between 350 nm and 370 nm. The samples should be exposure to NLT 1.2 million lux·hrs for cool white light and an integrated near UV energy of NLT 200 W·hr/m2.”
Photostability results should be organized in a summary table(s) that is suitable for regulatory submission according to the Common Technical Document (CTD) guidelines including data from the exposed and dark controls. The table may start with the header as shown below:
The testing results are presented in tabular form alongside the specifications. In the submission document, the photostability study data should be presented in the stability section 3.2.P.8, but stand apart from the long–term studies.
Alcami offers photostability studies at two laboratory sites:
These labs offer advanced analytical testing solutions for new drug entities and biopharmaceuticals, along with chemicals and animal health, medicated consumer health products, and generic drugs.
2 min read
In the WHO Technical Report Series, No. 953, 2009 Annex 2 Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products...
3 min read
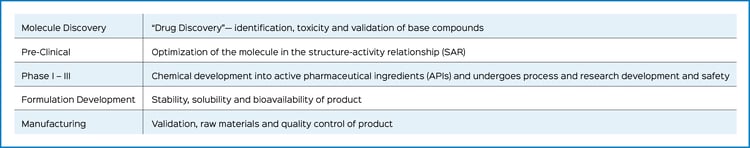
From molecule discovery through product release to market, quality attributes are carefully analyzed to support product success and protect patients.
3 min read