2 min read
Blog: The Impact of Stability Studies on APIs and Drug Products
Can you answer any of these questions?
From molecule discovery through product release to market, quality attributes are carefully analyzed to support product success and protect patients.
In the first step of the drug formulation process, drug discovery or the non-clinical phase, researchers investigate new understandings about a disease and design a product to stop or reverse its effects. Advanced technologies are explored, often to either target drug substances/active pharmaceutical ingredients (APIs) to specific locations within the body or to manipulate genetic material. During this phase, extensive research and development on how the finished product will be formulated also occurs.
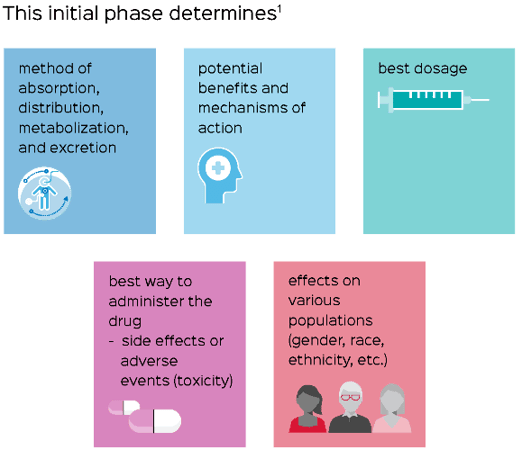
After this information is collected, the pre-clinical phase monitors the efficacy of the product and its potential to cause harm. These studies provide detailed information about the dosing and toxicity of the drug substance. Based on these findings coupled with meticulous research, a decision can be made whether or not to proceed to the clinical research phase.
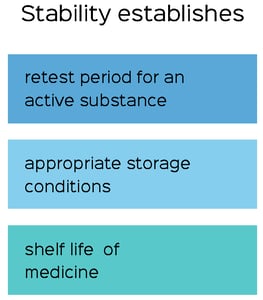
Stability becomes a major factor in Phase III, where it undergoes validation. Companies manufacturing finished goods or APIs conduct stability testing based on guidances outlined in the International Conference on Harmonisation (ICH). ICH focuses on the quality, safety, efficacy, and multidisciplinary structures of a product. As guided by the ICH, data should be provided on a minimum of three primary batches of drug product or API to establish product shelf life and suitable storage conditions. It is important to follow ICH guidelines when selecting batches, showing that “the overall quality of the batches of API placed on formal stability studies should be representative of the quality of the material to be made on a production scale.”
Once the batch selection is completed, a retest period would be applicable to all future batches manufactured under similar circumstances. This retest period determines whether the degree of variability from testing of the individual batches will affect the future production of a batch. If the product remains within certain specifications throughout the assigned retest period, the manufacturer can replicate the data of consistent batches.
The finished product goes through this guided testing to pinpoint particular degradation pathways. The ICH Q1A (R2) Stability Testing of a New Drug Substance and Product2 states: “The nature of any degradation relationship will determine whether the data should be transformed for linear regression analysis. Usually, the relationship can be represented by a linear, quadratic, or cubic function for an arithmetic or logarithmic scale.” Patient safety and ICH compliance are at the forefront of the development of the medicine during the stability process.
We, at Alcami, pledge to prioritize stability studies with every drug formulation, testing, and manufacturing procedure we conduct to help ensure we are working in a safe, connected way with our clients and their patients.
In conclusion, stability is an integral part of the drug development process. With every stability study, it is essential to focus on the quality attributes of the product – most importantly safety. Throughout this blog series, Alcami scientists discussed stability testing procedures, the “how” and “what” of degradation in the measurement of shelf life, the importance of storage conditions, and the necessity of following a medication’s recommended guidelines.
1“The Drug Development Process - Step 1: Discovery and Development.” U S Food and Drug Administration Home Page, Office of the Commissioner, 26 Mar. 2017, www.fda.gov/ForPatients/Approvals/Drugs/ucm405382.htm.
2 ICH, Q1A(R2) Stability Testing Of New Drug Substances (ICH, February 2003).
2 min read
Can you answer any of these questions?
3 min read
Stability testing is a fundamental part of the drug development process— upholding the quality of active pharmaceutical ingredients (APIs) and drug...
1 min read
The quality attributes that everyone strives for are safety, product efficacy and integrity, appropriate storage conditions, and shelf life.