2 min read
Execution and Reporting of a Photostability Study Protocol
The protocol design, execution, and reporting of the confirmatory photostability for API and pharmaceutical products will be discussed. ICH guidance...
Can you answer any of these questions?
Let’s be honest. Most people take these questions for granted. One quality attribute that all companies in the pharmaceutical field strive for is the safety of drug products.
The “Guidance for Industry” put in place by the U.S Food and Drug Administration (FDA) for drug stability guidelines states: “A drug product is considered unstable when the drug substance (active ingredient) loses sufficient potency to adversely affect the safety or efficacy of the drug or falls outside labeled specifications as shown by stability-indicating methods.”
In order to help ensure safety for all consumers, pharmaceutical drugs undergo different trials/phases including, but not limited to, the following:
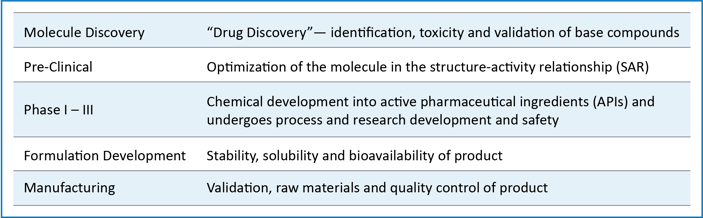
Contract development and manufacturing organizations (CDMOs) prioritize safety through each individual step from a stability standpoint during method development, formulation
How does stability help with the safety of pharmaceutical drugs?
Stability is one of the MOST important steps in an evaluation of drug safety and efficacy. Going back to the basics with quality attributes, stability plays a tremendous role in product development. When a product is placed in the International Conference on Harmonisation (ICH) conditions and evaluated through a spectrum of testing (physical, chemical, biological and microbiological) at different time intervals, we are able to monitor the degradation of the active pharmaceutical ingredient (API)/finished product. Degradation can develop at any point of these trials. In fact, even the smallest amount of degradation could cause adverse effects on consumers.
Stability’s purpose is to show how changes in light, temperature, pH, humidity, or just overall time can affect the API/finished product. Therefore, this trending of shelf life is essential in maintaining drug effectiveness, thus directly related to the product safety and toxicology of the product.
Not only is stability essential in the testing of pharmaceutical drugs, but the 21 CFR 211.94 of the current good manufacturing practices (cGMP) regulations also
Ultimately, the number one priority of any regulatory committee, and the number one priority at
2 min read
The protocol design, execution, and reporting of the confirmatory photostability for API and pharmaceutical products will be discussed. ICH guidance...
3 min read
1 min read
The quality attributes that everyone strives for are safety, product efficacy and integrity, appropriate storage conditions, and shelf life.