3 min read
Blog: Stability’s Role in the Drug Formulation Process
From molecule discovery through product release to market, quality attributes are carefully analyzed to support product success and protect patients.
The quality attributes that everyone strives for are safety, product efficacy and integrity, appropriate storage conditions, and shelf life.
Exactly how important is stability testing in the life of the active pharmaceutical ingredient (API) or drug product?
The US Pharmacopeia (USP) defines “stability” as the extent to which a product retains, within specific limits, and throughout its period of storage and use (i.e., its shelf life), the same properties and characteristics that it possessed at the time of its manufacture <USP1191>. The purpose of stability is to examine how the critical quality attributes of a drug substance vary with time under different environmental factors. These environmental factors include temperature, humidity, and light to establish a retest period (i.e. shelf life) for the drug substance and the recommended storage conditions. This process ensures that no physical, chemical, or microbiological changes affect the quality and integrity of the final products.
These storage conditions and shelf life testing in API and drug products directly take into account the intended markets based on the climate conditions. Derived from the mean kinetic temperature of any part of the world, four climatic zones have been distinguished for stability testing worldwide: Zone I-IV <ICH Q1A-Q1F>.
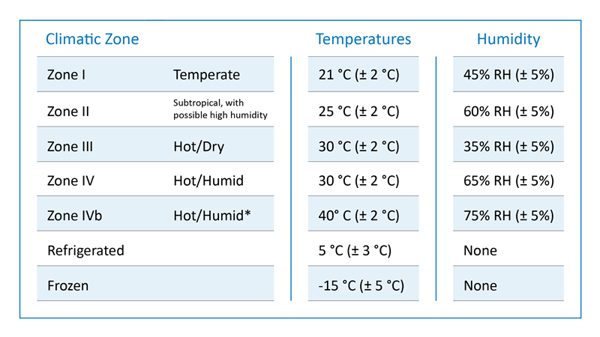
These climatic zones are simulated in chambers that involve temperature, humidity, and light under the guidance of the International Conference on Harmonisation (ICH) and U.S Food and Drug Administration (FDA) guidelines.
Through stability testing, pharmaceutical companies can help ensure that the product has the most suitable packaging or container closure for storage and distribution. And with the right storage and distribution methods in place, the quality of API and drug products are safeguarded.
3 min read
From molecule discovery through product release to market, quality attributes are carefully analyzed to support product success and protect patients.
2 min read
Can you answer any of these questions?
3 min read