4 min read
Beyond the Label: Four Challenges to Serialization
Many pharmaceutical manufacturers are concerned about label design and placement to accommodate a serialized label. These are, of course, very...
The implementation of the Drug Supply Chain Security Act (DSCSA) requirements poses many challenges. There can be an impact on productivity if your Serialization and Track/Trace systems are not prepared. Poor implementation plans can cause operational bottlenecks for several years as additional requirements are steadily implemented from now to 2024. As each system goes online, it will be necessary to “work out the bugs” and provide additional training for production and warehouse personnel.
Implementation costs may be an obstacle for smaller companies. Although the up-front costs will be fairly well defined, i.e., the purchase of software and hardware to create a serialization system, less defined are the costs to upgrade IT infrastructure. The setup of the serialization system and associated databases, ensuring compatibility between the serialization system software and other software management systems in a facility and long-term maintenance of databases may stress already stretched IT and production departments.
Serialization efforts must be scalable to support increasing DSCSA tracking requirements from now to 2024. A tremendous amount of tracking data will be generated at many levels. The technology components for unit level traceability are listed below.
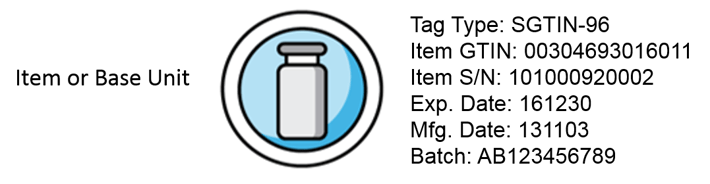
There is no current agreement on how the accumulated data and databases generated from the Track/Trace activities will be managed. Three possible models are:
Conclusion:
At this point in the implementation of DSCSA requirements, the greatest challenge appears to be how the data will be managed. Standardization of the data format has not been mandated by FDA but it seems that a standard format will be essential if data is to be easily shared among partners in a supply chain.
To learn more about serialization and how it affects your company, be sure to register for our on-demand webinar, “Get on Track with Serialization: Lessons Learned,” featuring Lee Murtagh, project leader for Alcami's serialization implementation.
*Please note that since the original publication of this blog entry, the serialization deadline has been extended to November 27, 2018.
4 min read
Many pharmaceutical manufacturers are concerned about label design and placement to accommodate a serialized label. These are, of course, very...
1 min read
According to the Food and Drug Administration and the Orphan Drug Designation program, orphan status applies to drugs and biologics defined as “those...
2 min read
The Food and Drug Administration (FDA) issued a draft guidance listing the enforcement of product identifiers under the Drug Supply Chain Security Act